The challenge of change without changing
During the Covid-19 pandemic, it has become apparent that globalization raises a number of issues. This includes supply disruptions as a result of restrictions implemented in order to reduce the spread of the virus. These disruptions can have serious consequences, especially for essential goods such as staple foods and healthcare products. Companies in these sectors are now looking for ways to further increase their security of supplies. Some supply issues, such as the ones caused by the scarcity of substances and raw material shortages, are complex and difficult to overcome on short notice, especially in the healthcare area, due to high safety standards. However, supply issues caused by available materials not reaching their destination due to geopolitical restrictions are easier to solve. Solutions generally include localizing the supply chain or finding alternative suppliers. Yet, in the case of healthcare products, another potentially challenging aspect to consider is the stringent and evolving regulatory landscape. Companies placing medical devices and packaged pharmaceuticals on the market need their products to comply with healthcare standards, no matter where their products are sold and no matter where they procure their raw materials. This can result in very complex and stringent change control and change management. For the plastics used in these medical applications, this can mean compliance with the relevant chapters of the International Organization for Standardization (ISO), the United States Pharmacopeia Class VI (USP VI) the European Pharmacopeia (EP 3.1), and the International Conference on Harmonization (ICH Q3D) standards and guidelines. Even regulations such as the updated European Union Medical Device Regulation (EU MDR) and In Vitro Diagnostic Regulation (IVDR) that are only valid for products sold in Europe, should preferably be considered from the start to ensure access to the European market.
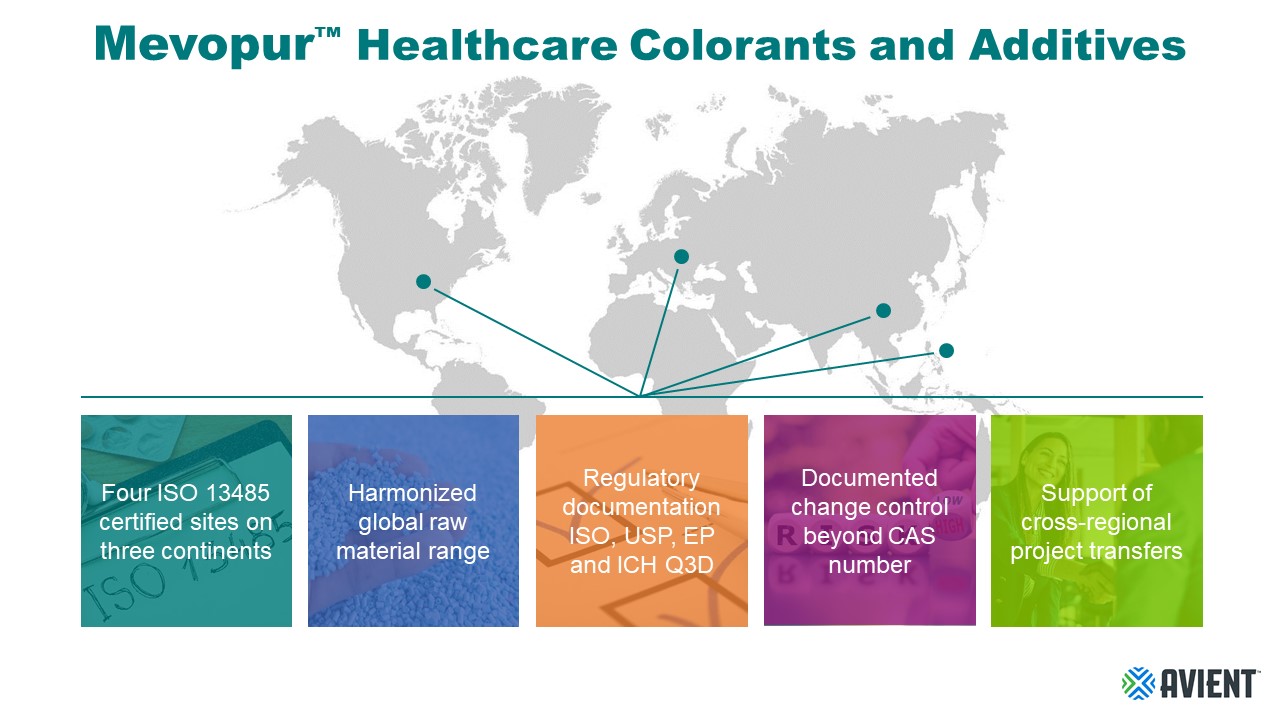
Avient’s Mevopur™ portfolio and risk mitigation concept can help healthcare manufacturers on the journey to increased security of supply for plastics used in their products. Mevopur colorants and functional additives are manufactured in four sites located in Europe, North America, and Asia. All four sites are ISO 13485 certified and use a harmonized raw material range as the base for globally available Mevopur standard portfolios and for custom formulations. The raw materials are pre-tested to the relevant chapters of ISO 10991, USP 661, USP Class VI, EP 3.1 and to the ICH Q3D guidelines to help manufacturers increase the certainty of regulatory compliance for their devices and packaging. Other regulatory compliance documentation is available upon request as well. This compliance testing, combined with the support of our healthcare experts, can make transfers of projects involving custom products from one region to another easier and faster. For healthcare companies, it means the selected custom colorant or functional additive can be procured from the Mevopur site most conveniently located for the converter, reducing the risk of supply disruption that can be caused by cross-border trade restrictions. Having multiple sites also means there is always a backup site producing to the same high-quality standards. All Mevopur production sites offer change control agreements beyond Chemical Abstracts Service (CAS) number.
Our Mevopur colorants and additive solutions can be supplied as concentrates or ready-to-use formulations. The concentrates need to be diluted with virgin polymer during the conversion process to achieve the right amount of color or additive in the plastic. The ready-to-use formulations already include the right amount of color and/or additive and can be converted as such without virgin polymer.